Abstract
Introduction: Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is an effective therapy for severe malignant diseases. Following allo-HSCT, donor T cells are the driving force for eradication of the remaining malignant cells known as graft-versus-tumor (GVT) effect. However, these alloreactive T cells are also responsible for induction of graft-versus-host disease (GVHD). To date, the role of the different Thelper (Th) subsets in the pathogenesis of GVHD is not completely understood. Interestingly, these subsets share expression of a transcription factor called Interferon Regulatory Factor 4 (IRF4), which is proposed as a master regulator of cell fate decision of T cells. This project aims to analyze the role of IRF4 in Th cell polarization during the development of GVHD.
Methods: In mixed lymphocyte reaction (MLR), we analyzed the proliferation capacity of CFSE-labelled IRF4-deficient (IRF4-/-) T cells upon allogeneic stimulation by LPS-induced dendritic cells (DC). For analyzing the impact of IRF4 in vivo, we used previously published complete MHC-mismatched murine GVHD model (Ullrich et al., J Clin Invest, 2018). Herein, we investigated the alloreactivity of the transplanted donor T cells towards GVHD target organs with focus on colonic tissue. Additionally, RNA sequencing of re-isolated and high purity FACS-sorted donor Th cells were performed to get a deep insight into the IRF4-mediated regulation of Th cell polarization.
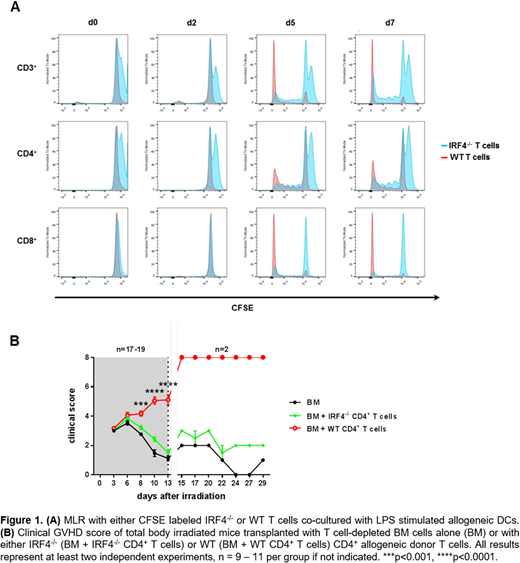
Results: In the MLR setting, reduced CFSE dilution indicated a diminished proliferative capacity of both CD4+ and CD8+ IRF4-/- T cells compared to the corresponding WT (IRF4+/+) T cell subsets upon allogeneic stimulation (Figure 1A). Furthermore, while alloreactive WT CD4+ T cells induced severe forms of GVHD in vivo, clinical GVHD symptoms of recipients transplanted with IRF4-/- CD4+ T cells were significantly reduced and these mice showed prolonged overall survival (Figure 1B). Analyzing the mechanism, we found that the frequency of in vivo circulating donorCD4+ IRF4-/- T cells was reduced compared to transplanted WT Th cells, especially in the GVHD target organs such as the colon. However, IRF4-/- Th cells persisted in spleen, lung and colon even if they showed a reduced proliferative capacity. In line with that, colonoscopy of mice transplanted with IRF4-deficient Th cells revealed a significant reduction of GVHD associated colitis. Transcriptome analysis of re-isolated and high purity FACS-sorted donor Th cells depicted an altered gene expression profile in donor IRF4-/- Th cells compared to donor WT Th cells. Specifically, master regulators of Th cell subsets like T-bet (Th1), RORγt (Th17) and to some amount also GATA-3 (Th2) were downregulated in donor IRF4-/- Th cells whereas FoxP3, the master regulator of regulatory T cells (Treg cells), was significantly upregulated. Along the same line cytokines associated with Th1, Th2 and Th17 cell subsets such as IFN-γ, IL-21, IL-6 and IL-13 were also significantly downregulated. Besides genes that are associated with Treg cell function like Helios, FR4 (folate receptor 4) and Neuropilin 1, a transcriptional repressor, Bach2, which regulates the formation of Treg cells and suppresses Th1, Th2 and Th17 subset differentiation was highly upregulated (Tsukumo et al., Proc Natl Acad Sci U S A, 2013; Kim et al., J Immunol, 2014 ; Roychoudhuri et al., Nature, 2013 ; Vahedi et al., Nature, 2015). Along with the upregulation of Bach2 and the significant downregulation of Blimp1, another transcriptional repressor involved in T cell homeostasis and function as well as direct target of Bach2, we hypothesize that IRF4 might compete with BACH2 for the binding to BATF. These hypotheses also rely on our previous finding of BATF as critical mediator of GVHD colitis and are currently under further evaluation (Ullrich et al., J Clin Invest, 2018).
Conclusion: In summary, our results indicate that IRF4 plays a key role in regulation of the Th cell polarization and therefore also in the development of GVHD. Thus, IRF4 in its interplay with BATF might be considered as a clinically relevant target for GVHD therapy.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal